In
the SF6 circuit breaker the current carrying contacts operate in sulphur
hexafluoride gas is known as an SF6 circuit breaker. It is an excellent
insulating property and high electro-negativity. It can be understood that,
high affinity of absorbing free electron. The negative ion is formed when a
free electron collides with the SF6 gas molecule; it is absorbed by that gas
molecule. The two different ways of attachment of electron with SF6 gas
molecules are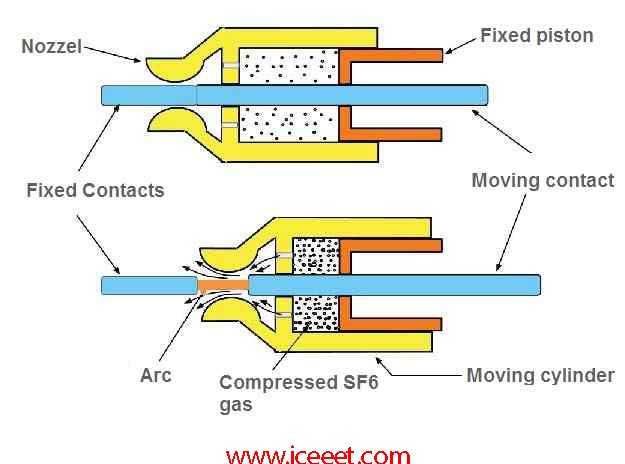
The negative ions which are formed will be much heavier than a free electron. Therefore, when compared with other common gases overall mobility of the charged particle in the SF6 gas is much less. The mobility of charged particles is majorly responsible for conducting current through a gas. Hence, for heavier and less mobile charged particles in SF6 gas, it acquires very high dielectric strength. This gas good heat transfer property because of low gaseous viscosity. SF6 is 100 times more effective in arc quenching media than air circuit breaker. It is used for both medium and high voltage electrical power system from 33KV to 800KV.
Types of SF6 Circuit Breaker
Single
interrupter SF6 circuit breaker applied up to 220
Two
interrupter SF6 circuit breaker applied up to 400
Four
interrupter SF6 circuit breaker applied up to 715V
Related Articles
Lesson meta keywords and meta description:
Write a public review